CAR-T Virus free gene delivery, integration and expansion technologies, Quantum Engines™

GenomeFrontier Therapeutics, Inc. – committed to the development of advanced, affordable and accessible immune cell therapies for efficacious cancer treatments
Defining unmet needs in gene therapy
• Viral vector is currently the most common vehicle for gene therapy due to its high gene delivery efficiency.
• However, use of viral vectors are associated with several disadvantages including (1) virus-associated safety risks, (2) high manufacturing costs, and (3) limited payload capacity of viral vectors, which severely restrict the repertoire of genes that can be encoded.
• A virus-free vector obviates these shortcomings. It is safer than viral vectors due to lower immunogenicity and lower risk of genome toxicity. Moreover, virus-free vector has the capacity to carry larger fragments of genetic material and the manufacturing costs of virus-free vectors are also markedly lower than those required for viral vector production.
• However, despite the advantages of virus-free vectors, it remains a challenge to efficiently deliver these vectors into immune cells such as T cells and also to efficiently integrate genes into the genome for stable expression. Electroporation is currently the most efficient method for delivery of virus-free vectors into immune cells, but electroporated cells become fragile with markedly reduced viability and expansion capacity.
• Therefore, there is clearly an unmet need for major improvements in this area of gene therapy.
Quantum EngineTM addresses the unmet needs
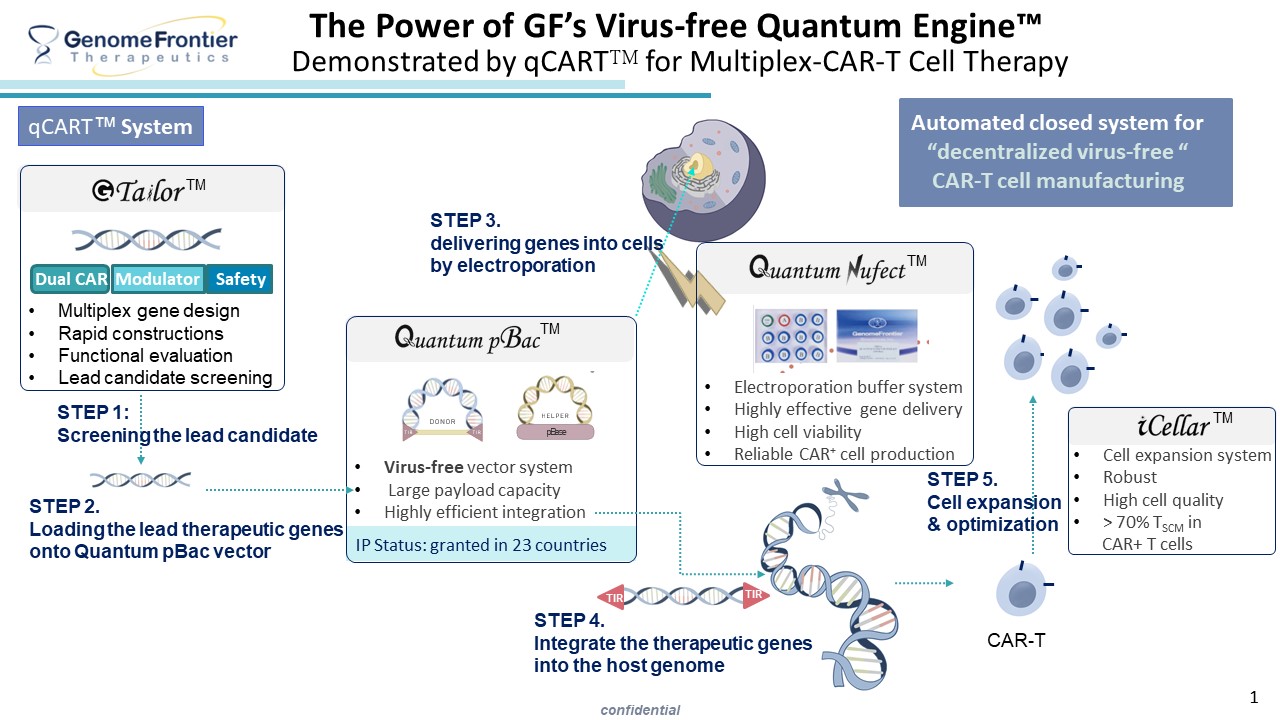
• GenomeFrontier Therapeutics, Inc. (GF) addresses these unmet needs of conventional gene therapy by integrating four platforms: Quantum Nufect™, Quantum pBac™, iCellar™ and G-Tailor™, which improve vector delivery, therapeutic gene integration, cell expansion capacity and gene design, respectively. Synergistically, these platforms form the Quantum Engine™ for producing virus-free, gene-modified cells optimal for immune therapy.
• Taiwan and international (PCT) applications covering a total of 12 jurisdictions including United States, China, Japan, Korea, Australia, Europe, Canada, Malaysia, Singapore, Mexico, New Zealand and Taiwan have been filed to protect Quantum Engine™’s core technologies. Notices of allowance/patents have already been issued in Europe, Malaysia, Korea, Canada, Australia, Mexico and in Taiwan.
• GF has successfully utilized the Quantum Engine™ to produce dual-targeting (CD19, CD20) CAR-T cells that contain a safety switch control gene (GF-CART01). As shown in Figure 1 below, when compared with CAR-T cells that have been produced using the lentivirus system, Quantum Engine™ produces a markedly higher % (median of 75%) of CAR+ T cells that are also more robustly expanded (median of 1454 fold increase). Quantum Engine™ also produces a majority population of TSCM cells (median of 82%) that are thought to represent the most therapeutically efficacious T cell population.
• The company plans to utilize GF-CART01 cells for the treatment of patients with B cell malignancies. IND-enabling preparation of GF-CART01 cells including GMP/GTP production, pre-clinical studies and pre-IND consultation is planned for 2021. Filings of IND applications in the United States and in Taiwan are planned for 2022.
Adoptive transfer of GF-CART01 cells dose-dependently eradicates Raji tumors in mice
• As shown in Figure 2, GF-CART01 cells produced from donors PBMC-40 and PBMC-41 and adoptively injected into Raji-bearing mice completely eradicated Raji tumor cells by Day 21 post transfer. The treated mice remained tumor free for at least 91 days, end of the study. GF-CART01 cells’ anti-tumor efficacy was also dose-dependent, as can be demonstrated in IVIS images of mice injected with low, medium and high doses of PBMC-41 GF-CART01 cells. Importantly, even with tumor rechallenge (red boxes), GF-CART01mid and high dose groups remained tumor free.
Additional ongoing endeavors to broaden the repertoire of “GPS-guided” immune cells,
and to provide affordable, accessible and effective immune cell therapies
• The company continues to pursue R&D projects to value create for Quantum EngineTM and all of its associated platforms and products.
• GF is at the development stage for the next CAR-/TCR- T system for production of allogenicv and CAR-/TCR-NK and CAR-/TCR-gamma-delta T cells.
• The company is also focusing on the automation and decentralization of CAR-T cell manufacture with the long-term goal of providing affordable and effective CAR-T cell therapies that are widely accessible to patients in need. We are in collaboration with ADVA Bio to develop a desktop automatic and closed virus-free CAR-T cell production system.
線上展網址:
https://tievirtual.twtm.com.tw/iframe/4cb17a86-3434-4995-a329-c2ac2351a272?group=f36efffd-9ee6-4962-aa09-72f6797d2449&lang=en
GenomeFrontier Therapeutics, Inc. leverages a combination of four non-viral gene modification platforms to build a modular production system for gene-modified cell products. Using the Quantum pBac™ non-viral vector, along with the in-house developed Quantum Nufect™ electroporation solution and Quantum Booster™ for CAR-T cell expansion, the company has developed the qCART™ system. Additionally, its proprietary G-Tailor™ platform enables rapid assembly of designed gene fragments for application across multiple systems.
Technology maturity:Prototype
Exhibiting purpose:Product promotion、Display of scientific results
Trading preferences:Technical license/cooperation、Negotiate by self
*Organization
*Name
*Phone
*Main Purpose
*Discuss Further
*Job Category
*Overall Rating
*Favorite Area
*Key Tech Focus
*Willing to Receive Updates?
Other Suggestions
Coming soon!