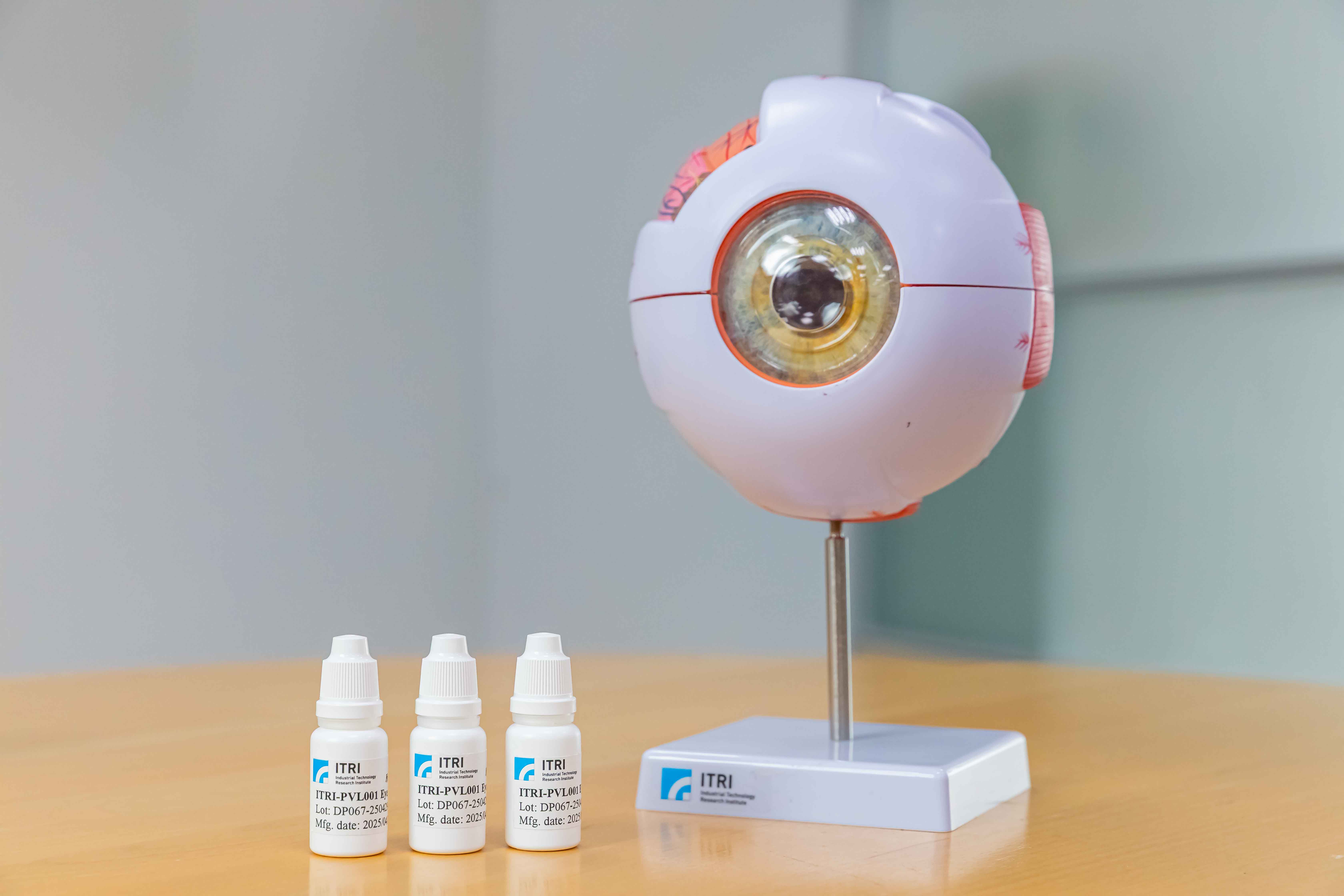
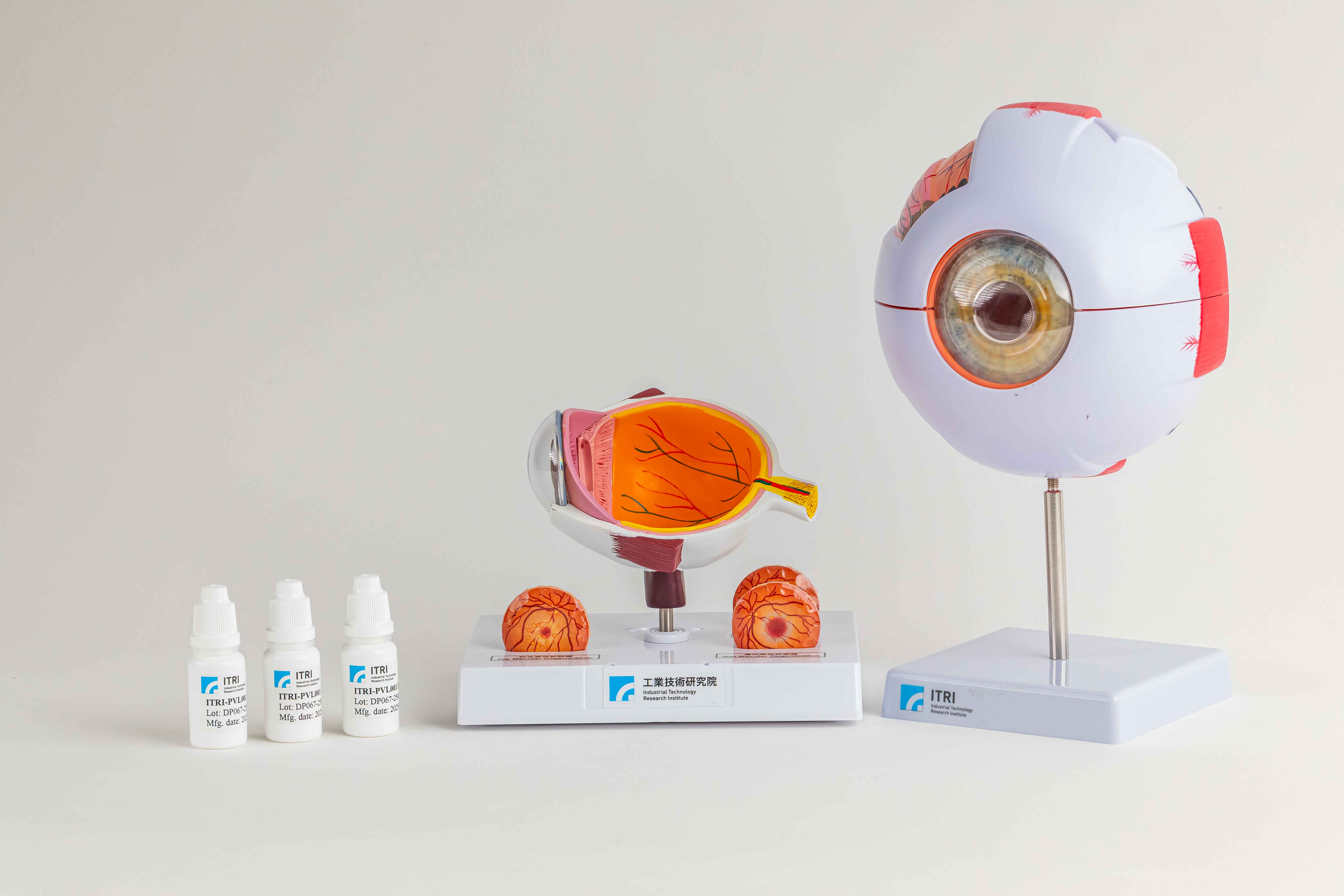



ITRI’s 505(b)(2) eye drop offers a non-invasive, self-administered therapy for intermediate to late-stage age-related macular degeneration (AMD), utilizing a patented formulation to enhance drug exposure in posterior ocular tissues. Preclinical studies show superior efficacy over intravitreal Risuteganib in preserving photoreceptor integrity and supporting retinal pigment epithelium (RPE) function by reducing endoplasmic reticulum (ER) stress and sub-RPE lipid accumulation. Animal studies also confirm good ocular safety, supporting strong clinical potential.
ITRI is a world-leading applied technology research institute with more than 6,000 outstanding employees. Its mission is to drive industrial development, create economic value, and enhance social well-being through technology R&D. Founded in 1973, it pioneered in IC development and started to nurture new tech ventures and deliver its R&D results to industries. ITRI has set up and incubated companies such as TSMC, UMC, Taiwan Mask Corp., Epistar Corp., Mirle Automation Corp., and Taiwan Biomaterial Co.
Name:Yu-Wen Lo
Phone:(03)5743976
Address:195, Sec. 4, Chung Hsing Rd., Chutung, Hsinchu, Taiwan 31040, R.O.C.
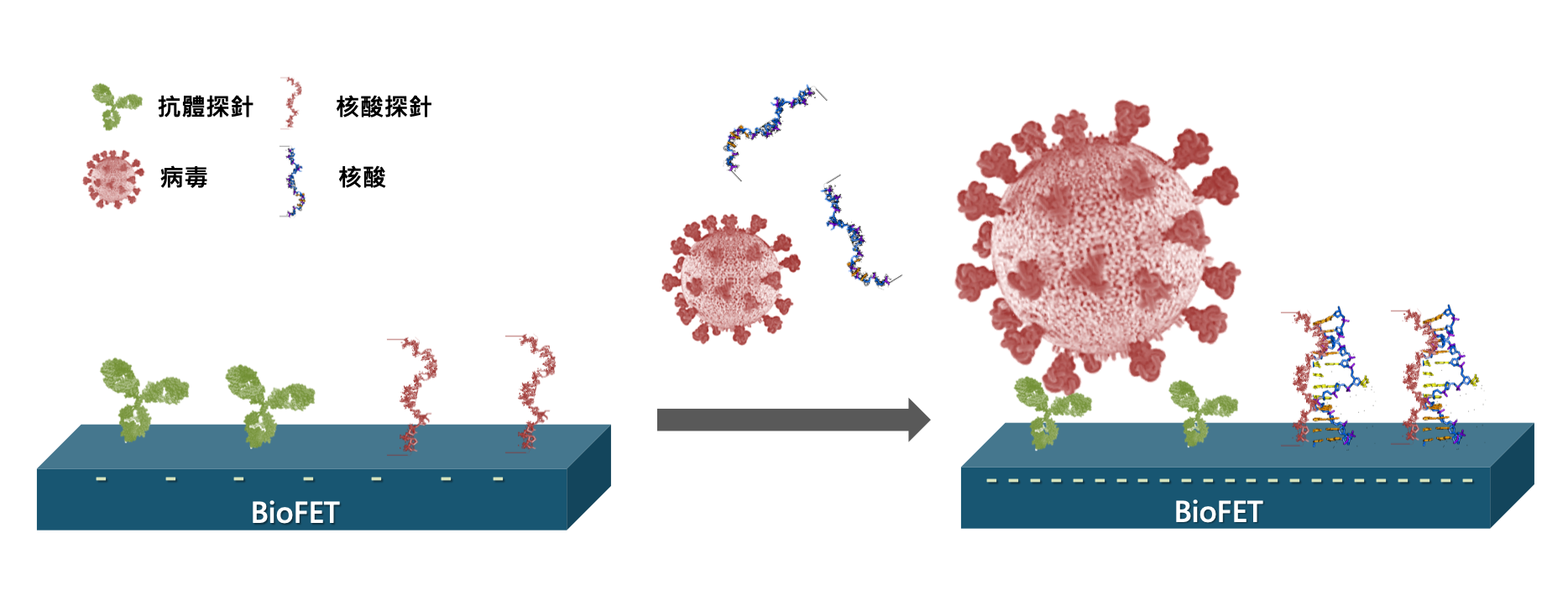
Ultrahigh-Sensitive Detection Platform Powered by Semiconductor-based Biosensor for the evaluation of Treatment on Acute Myeloid Leukemia

A Novel Eye Drop Formulation for Wet AMD Treatment
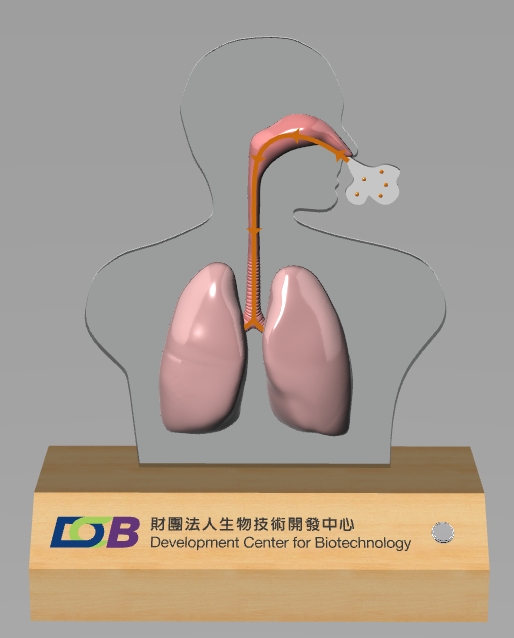
Platform for Oligonucleotide Drug Production (Nucleic Acid Drugs for the Treatment of Pulmonary Fibrosis)
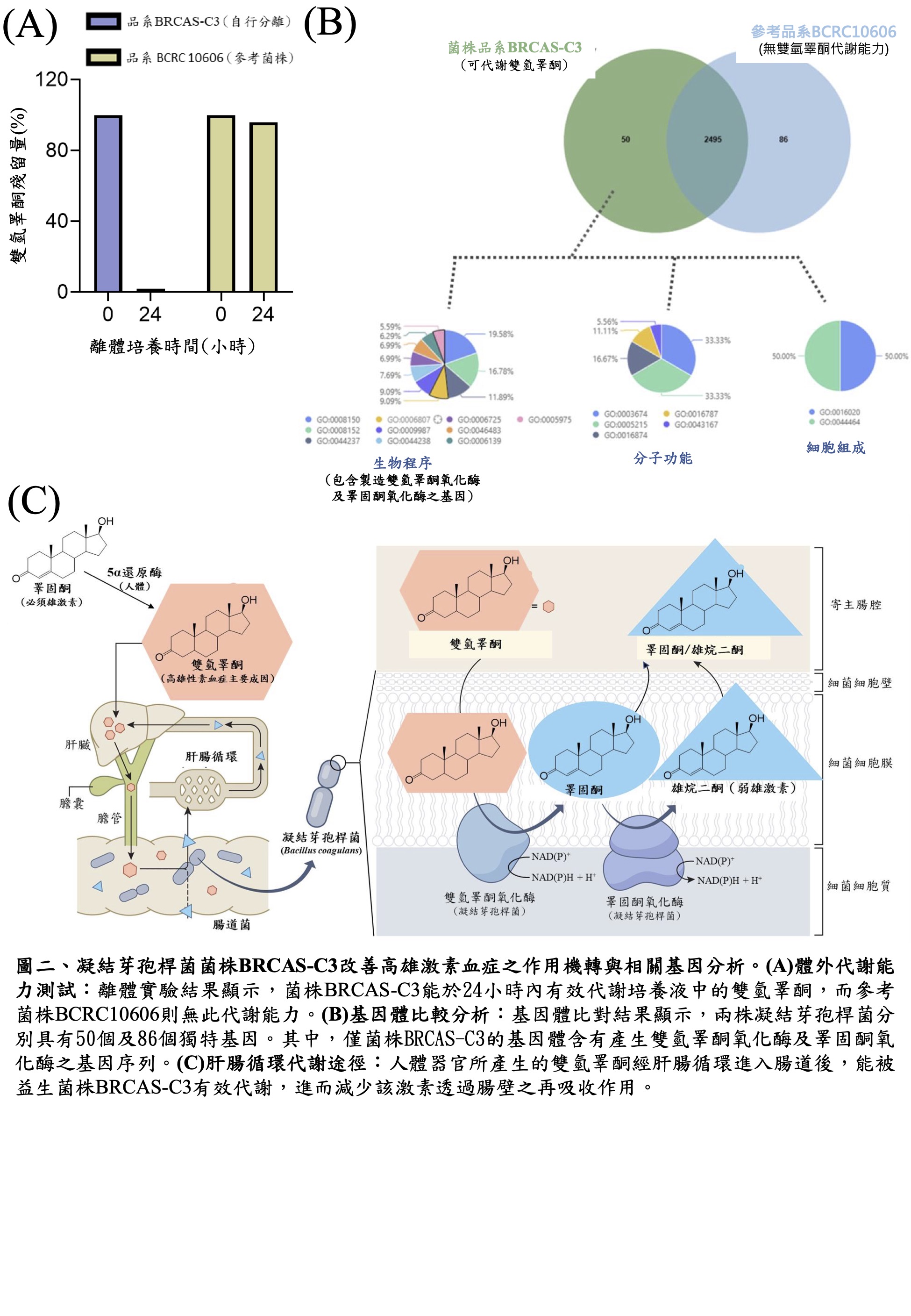
Innovative Development of Androgen-Metabolizing Probiotics: Functional Gut Microbiome Therapy for Hyperandrogenism Treatment
Technology maturity:Mass production
Exhibiting purpose:Display of scientific results
Trading preferences:Negotiate by self
*Organization
*Name
*Phone
*Main Purpose
*Discuss Further
*Job Category
*Overall Rating
*Favorite Area
*Key Tech Focus
*Willing to Receive Updates?
Other Suggestions
Coming soon!