An effective and safe first-in-class new drug for abdominal pain in irritabl e bowel syndrome


Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder cha
racterized by recurrent abdominal pain and changes in bowel habits. Alth
ough IBS accounts for 20-40% of gastroenterology outpatients, there is n
o satisfactory drug for the treatment of IBS abdominal pain and it is still a
highly unmet need. We found the serotonin type 7 receptor (5-HT7R) is a
new target for the treatment of IBS abdominal pain. After drug design and
lead optimization, a safe and effective drug candidate DC105 was selectedfor preclinical studies, and an investigational new drug application is expe
cted to be filed in 2024.
This technique consists of three parts-
1. Discovery of novel mechanism and target: Colon biopsy specimens fro
m IBS patients and healthy subjects were collected. Higher expression of 5
-HT7R was found in the specimens from IBS patient than that of healthy s
ubjects. Human nerve cell line SH-SY5Y was used to confirm that adding i
ntestinal tissue sterile supernatant, 5-HT, and neurotrophic factors can pr
omote nerve fiber elongation, while pretreatment of DC105 or 5-HT7R ge
ne silencing can reduce the length of nerve fibers. There is a positive feed
back relationship between 5-HT and neurotrophic factors, which can caus
e excessive growth of nerve fibers by 5-HT7R activation. Thus, 5-HT7R ant
agonist is a promising treatment for IBS pain.
2. Establishment of a platform for evaluation of analgesic activity: The pos
t-infection with water avoidance stress GW mice and the post-inflammatio
n PT mice models were established. The visceral pain in mice and the drug
activity to inhibit hyperalgesia were determined by measuring the visceral
motor responses stimulated by colorectal distension.
3. Development of a first-in-class drug for IBS abdominal pain: DC105 is a
highly specific 5-HT7R antagonist with suitable drug-like properties and s
afety. After oral administration of DC-105, the visceral hypersensitivity wa
s effectively reduced without affecting normal
學研單位
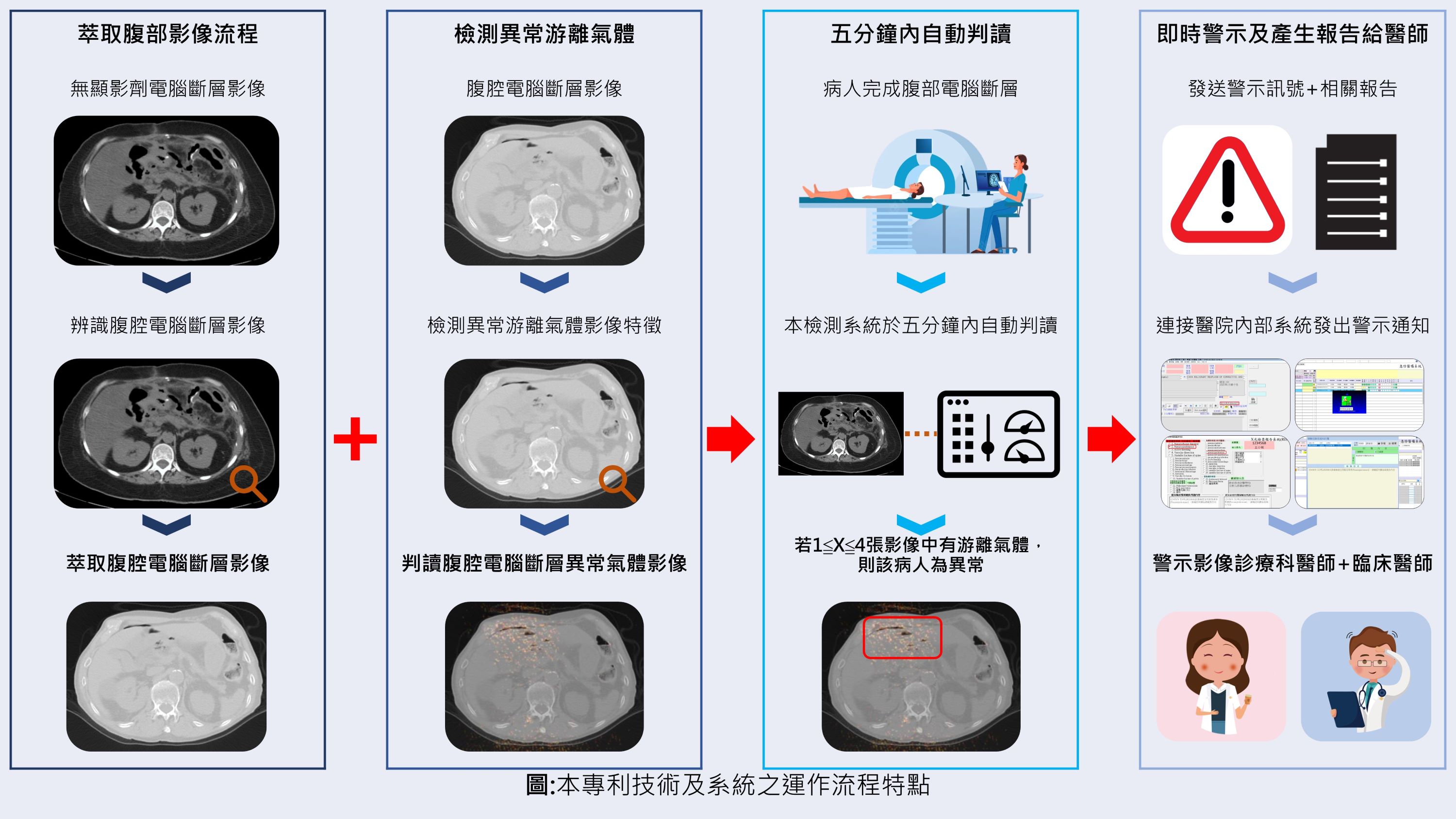
A deep learning-powered novel artificial intelligence algorithm and syste m to assist in the identification of pneumoperitoneum on abdominal com puted tomography
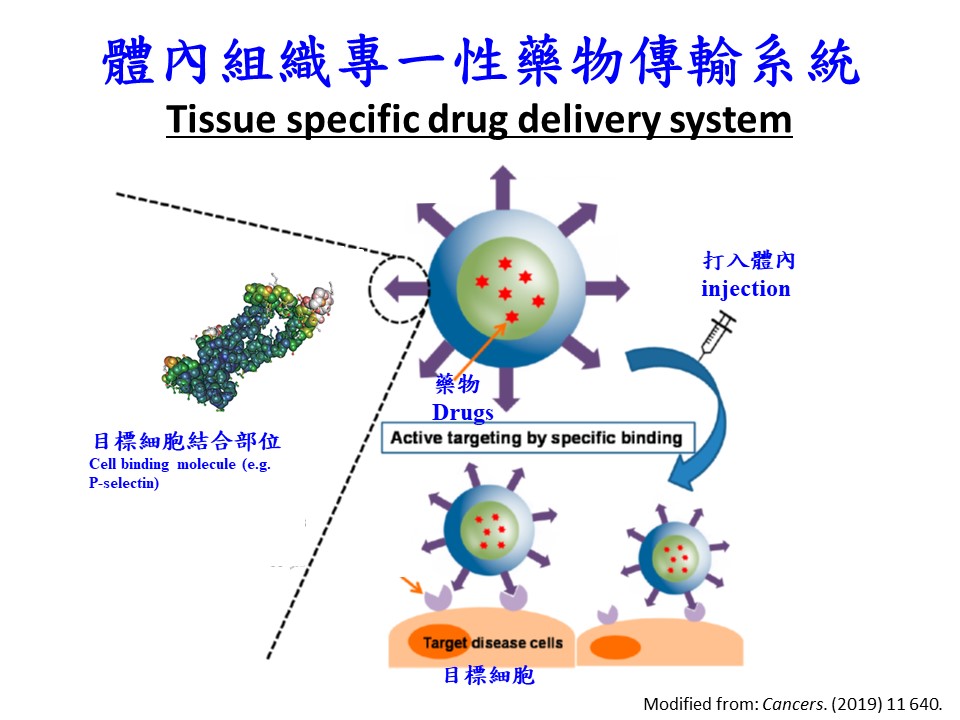
Vesicles comprising lectins expressed on the surface and methods of use thereof to deliver an agent to autophagic and apoptotic cells
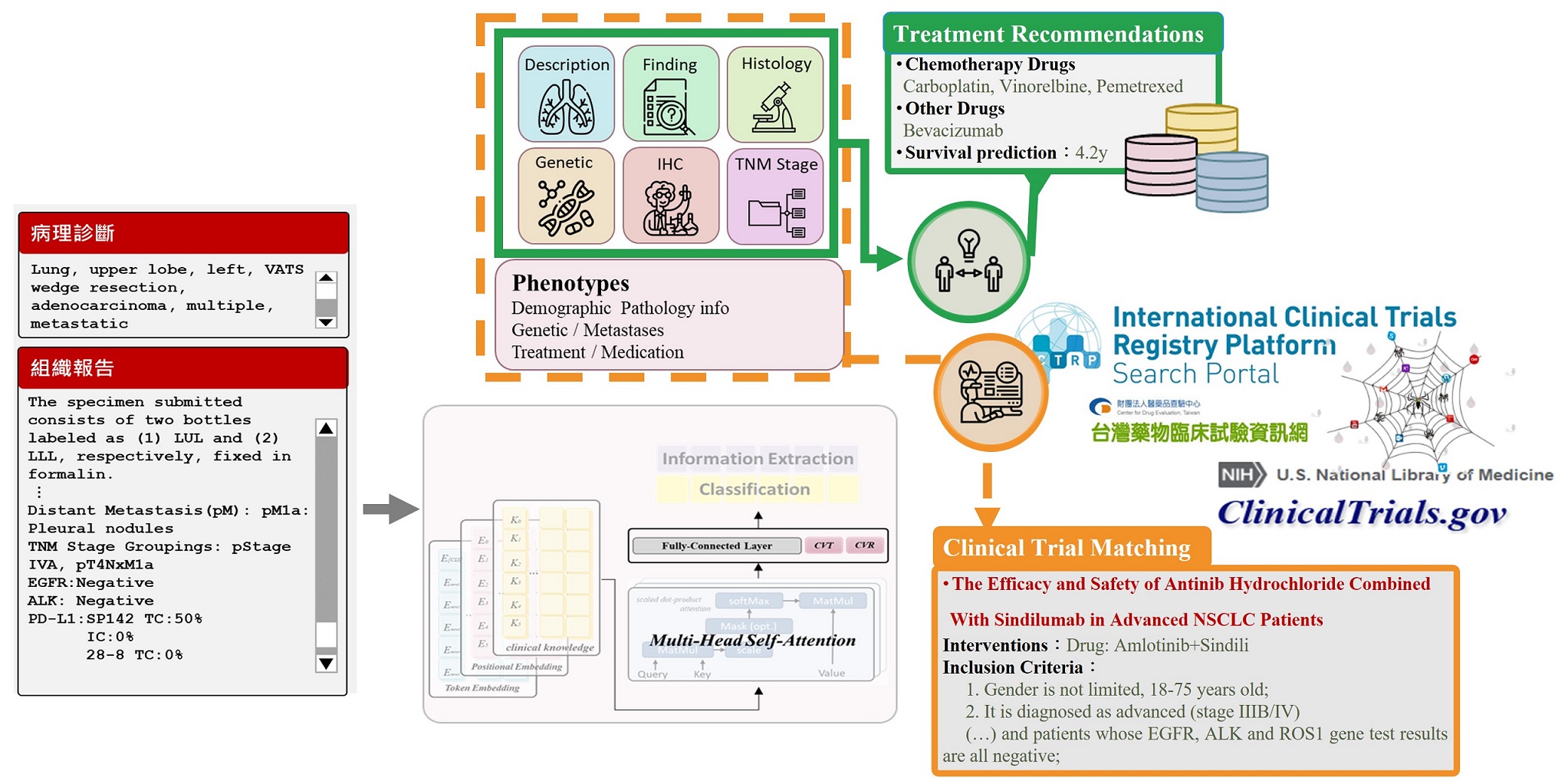
Using Generative Deep Learning to Predict Drug Response and Survival, a nd Automatically Match Clinical Trials for Advanced Lung Cancer.
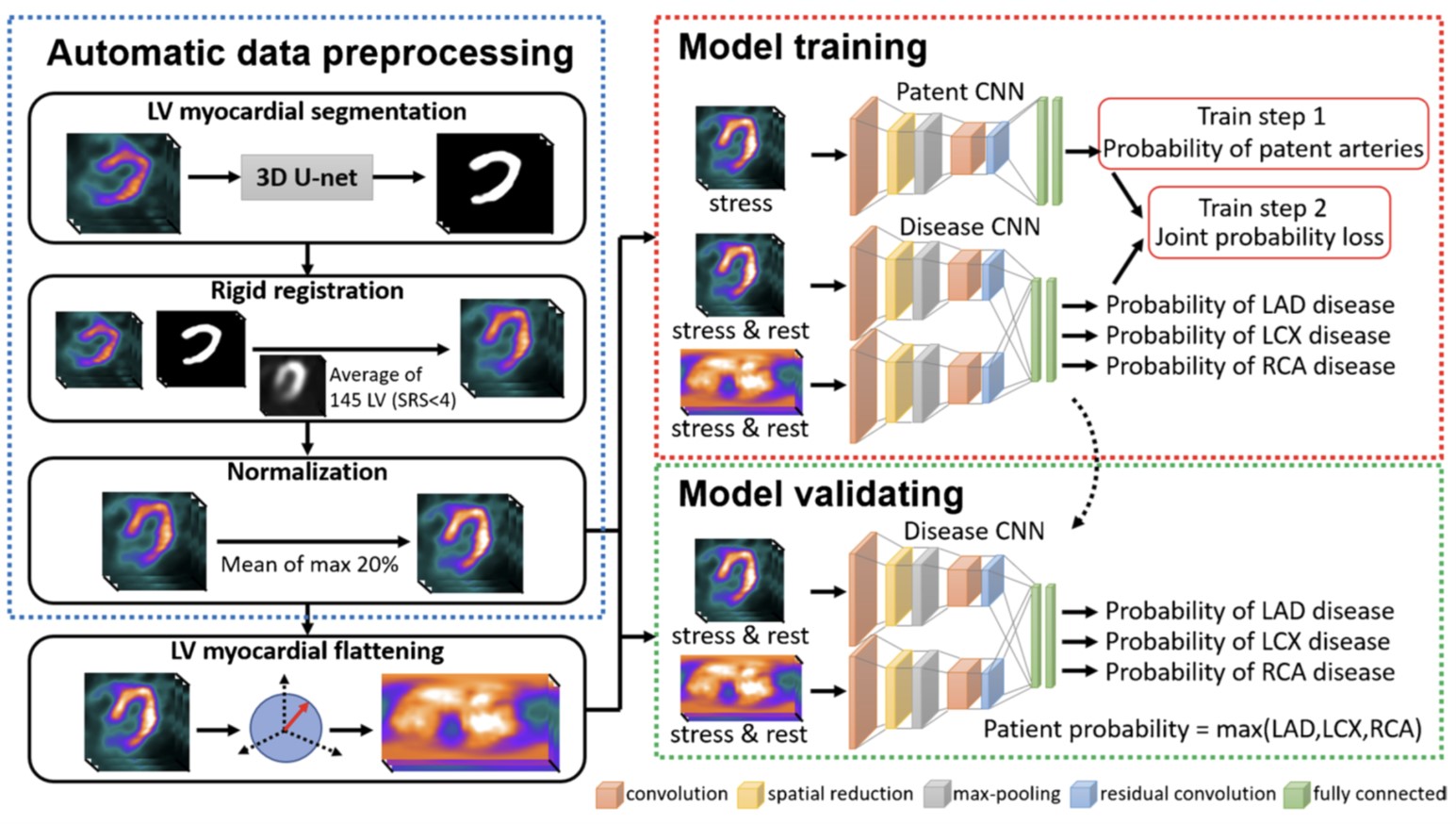
One Model Fit All: Revolutionary One-Stop Shop System for Predicting Co ronary Stenosis without Normal Database in Myocardial Perfusion Imagin g
Technology maturity:Experiment stage
Exhibiting purpose:Display of scientific results
Trading preferences:Negotiate by self
Coming soon!