Hepatitis B virus detection platforms for novel biomarkers and clinical ap plications


Chronic hepatitis B virus infection is among the major public health proble
ms worldwide. Up to now, two billion people have been infected with HBV,
and more than 300 million have developed chronic hepatitis. They are at h
igh risks of advanced liver diseases, including cirrhosis and hepatocellular
carcinoma (HCC). HCC is one of the world's top ten leading causes of dea
th, especially in countries where hepatitis B is prevalent, such as Asian and
African countries. Therefore, effectively monitoring the treatment effects
of HBV is essential for evaluating the therapeutic strategies and preventio
n of HCC. Currently, the major HBV drugs are the nucleotide/nucleoside a
nalogs, which inhibit the reverse transcription process of viral genes, how
ever, they cannot eradicate the viral covalently closed circular (ccc) DNA a
nd its transcript pre-genomic RNA, therefore the risks of hepatitis recurre
nce after cessation of treatments are high. The cccDNA is a key predictive biomarker of viral reactivation. Our research team focuses on developing
high-sensitivity and -specificity quantitation methods to monitor the HBV
treatment efficacies. We used digital PCR (dPCR) technology to establish t
he simultaneous quantitative detection of HBV DNA load and cccDNA as
predictive biomarkers for hepatitis recurrence and HCC. Our performance
s are:
1. Development of the purification protocols of liver tissue and serum ccc
DNA, and the single-tube reaction of T5 and T7 exonucleases to improve t
he specificity of cccDNA detection, shorten the operation time, and simpli
fy operations.
2. Development of a dPCR system for quantifying HBV DNA, effectively im
proving the sensitivity of HBV detection.
3. Development of the LDTS test kit for a simultaneous quantification of H
BV viral load and cccDNA by dPCR.4. Clinical tests in HBV cohorts through collaborations with the NCKU Hospital to screen out low-titer HBV-positive carriers. Large-scale screening for the low-titer HBV carriers by dPCRis
National Cheng Kung University (NCKU) envisions its campus as a place that nurtures imagination, grounded in solid academic research and high-quality learning. The university is committed to fostering urban development and global sustainability as part of its centennial mission. By breaking institutional barriers and strengthening interdisciplinary teaching and research, NCKU encourages students to recognize social issues, produce research that meets societal needs, and actively engage in solving global challenges—reflecting its responsibility as a leading university.
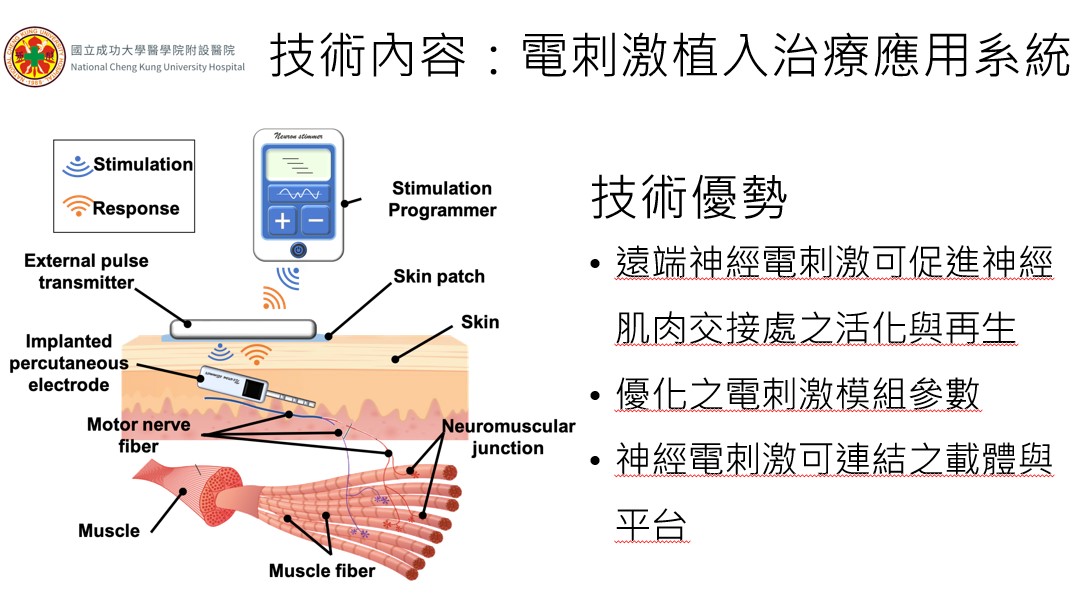
Development of an Implantable Bioelectronic Platform for the Peripheral Neuromuscular System Regeneration
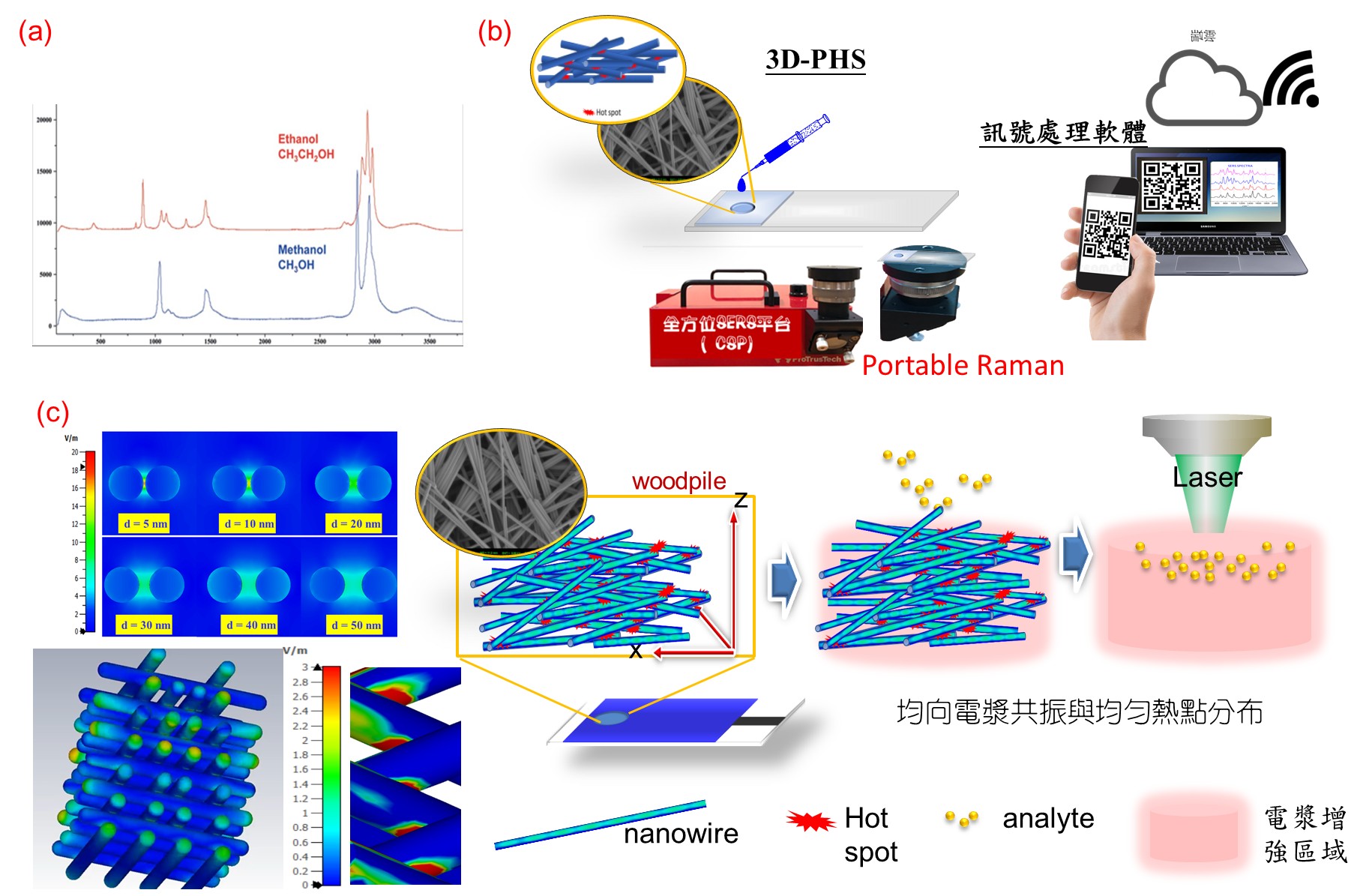
Comprehensive SERS Detection Platform (CSDP) for Biomedicine and Foo d Safety Detection
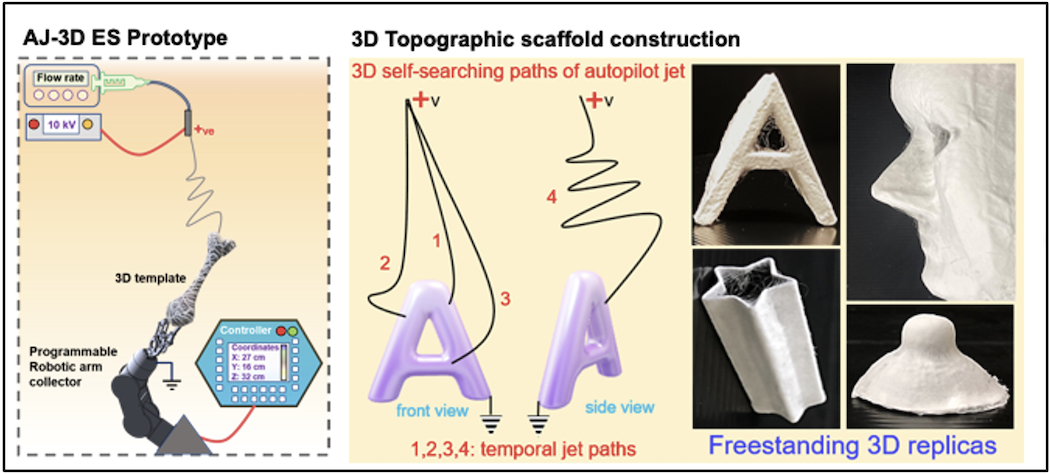
Novel Autopilot Jet-Based Electrospinning for Fabricating Functional 3D Scaffolds for Tissue Engineering and Regenerative Medicine Applications

MRI Compatibility Testing and Evaluation Platform for Medical Device and Implants
Technology maturity:Experiment stage
Exhibiting purpose:Display of scientific results
Trading preferences:Negotiate by self
*Organization
*Name
*Phone
*Main Purpose
*Discuss Further
*Job Category
*Overall Rating
*Favorite Area
*Key Tech Focus
*Willing to Receive Updates?
Other Suggestions
Coming soon!